Electric Charges Explained: Neutral Atoms, Attraction, Repulsion, and Coulomb’s Law
- PLC Play Ground
- 0
- Posted on
Electricity begins at the atomic level. To understand how electric charge works, we must look at the structure of atoms and how electrons and protons create positive, negative, or neutral states.
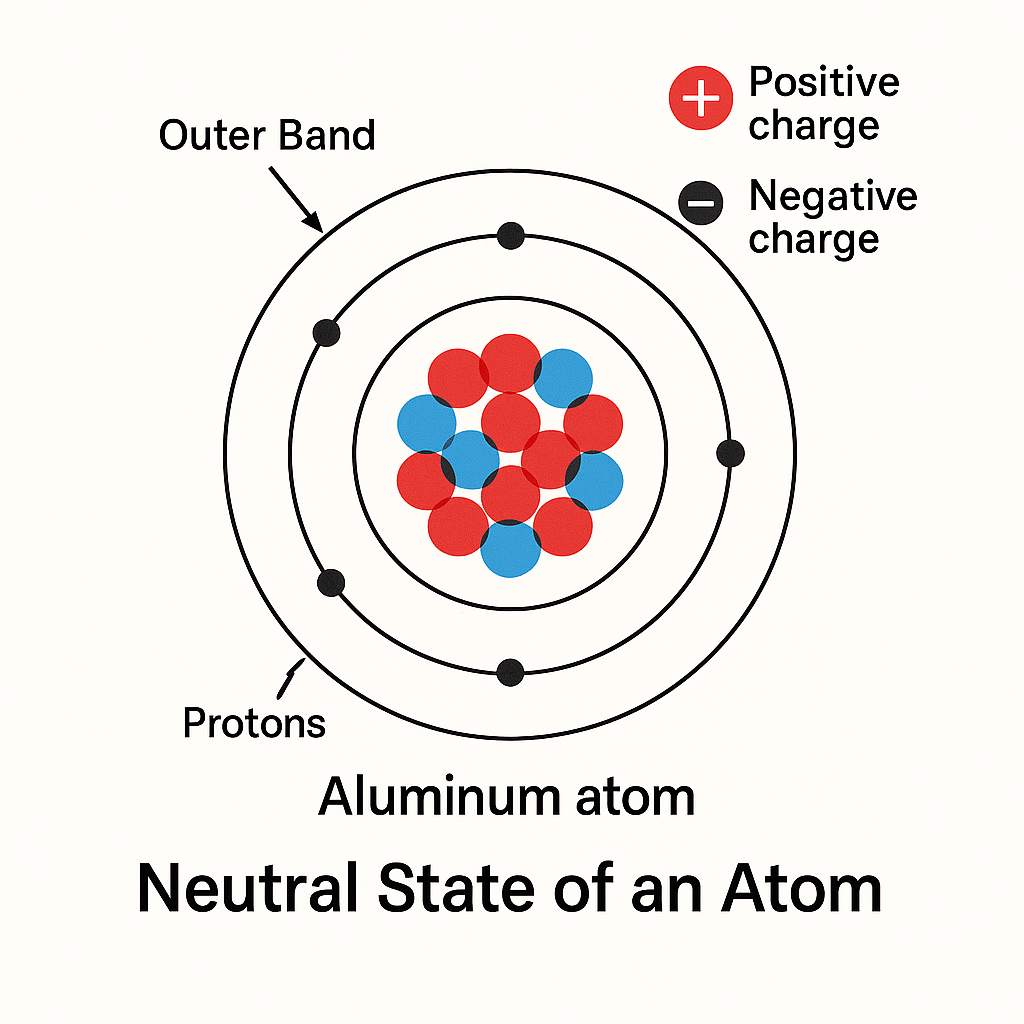
Neutral State of an Atom
Atoms are identified by the number of electrons orbiting the nucleus and the number of protons inside the nucleus.
- A hydrogen atom has 1 electron and 1 proton.
- An aluminum atom has 13 electrons and 13 protons.
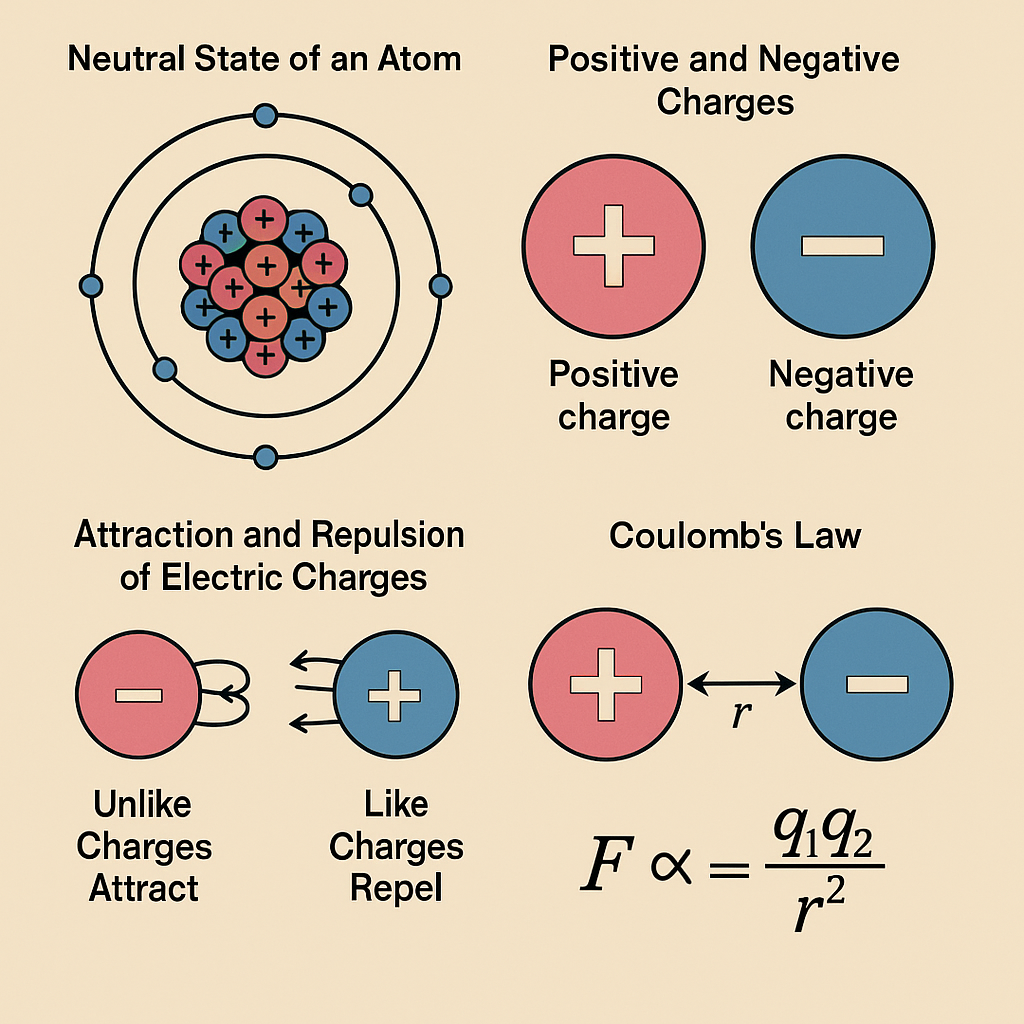
When an atom has an equal number of electrons and protons, it is electrically neutral.

Positive and Negative Charges
Electrons located in the outer band of an atom can be easily displaced when an external force is applied. When electrons move:
- The place where electrons leave gains a positive charge (more protons than electrons).
- The place where electrons collect gains a negative charge (excess electrons).
In other words:
- Positive charge = lack of electrons
- Negative charge = excess of electrons
- Number of protons never changes
Attraction and Repulsion of Electric Charges
The familiar phrase “opposites attract” is quite literal in electricity.
Each charged object generates an invisible electric field. This field can interact with other charged bodies.
- Like charges repel each other
(positive ↔ positive or negative ↔ negative) - Unlike charges attract
(positive ↔ negative)
These electric fields are often illustrated with lines of force, which:
- Leave a positive charge
- Enter a negative charge
These lines visually represent the direction and behavior of the electric field responsible for attraction or repulsion.
Coulomb’s Law
In the 18th century, French scientist Charles A. Coulomb studied the forces around charged bodies. His work led to what we now call Coulomb’s Law, which states:
The force between two charged objects is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
In simple terms:
- Stronger charges → stronger force
- Greater distance → weaker force
Coulomb’s Law helps us quantify the electrical attraction or repulsion between charged objects and is fundamental to understanding electrical behavior in circuits, materials, and even everyday static electricity.
